Based on what you already know about photosynthesis, develop a testable hypothesis to explain the influence of an increase in light intensity on the photosynthetic rate in tomato leaves.
- Choosing a Leaf and Measuring Leaf Surface Area
To set up each experiment, first choose the leaf you are interested in studying. Leaves from six different plants are available in LeafLab. The plants available to you are tomato (C3 plant), corn (C4 plant), two different clones of goldenrod (one favors sunny conditions, the other favors shade), and two types of fescue grass that differ by their number of chromosomes.
- Input Controls
The Input Controls view allows you to change several conditions in the chamber. These include gas flow into the leaf chamber, temperature, carbon dioxide concentration, light intensity, and light quality. Gas flow is measured in milliliters of gas entering the chamber per minute (ml/min). The default value for gas flow is "off." Temperature is measured in degrees Celsius (°C). The default value for temperature is set at 25°C, the temperature of a warm room (80.6°F), which is a fairly comfortable temperature for most plants living in a typical greenhouse. Carbon dioxide concentration is measured in parts per million (ppm). The default value is 350.0 ppm. This value approximates the normal atmospheric concentration of carbon dioxide. Light intensity is measured in micromoles of light photons released per square meter per second (
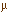 mol/m2/s). The default value is that the light is turned off
(0
mol/m2/s). The default value is that the light is turned off
(0 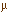 mol/m2/s). Unless you are performing an experiment to measure
photosynthetic rates under darkness, you must always adjust light
intensity before you can collect data. The light filters control
allows you to determine which wavelengths of light you would like
the leaf to be exposed to. The default is no filter; thus, white
(visible) light is striking the leaf.
mol/m2/s). Unless you are performing an experiment to measure
photosynthetic rates under darkness, you must always adjust light
intensity before you can collect data. The light filters control
allows you to determine which wavelengths of light you would like
the leaf to be exposed to. The default is no filter; thus, white
(visible) light is striking the leaf.
- Analyzing Data
To prepare your data for analysis, switch to the Prepare Data view.
- Plotting Data
Plotting data from the calculations that you generate is important for understanding the results of your experiments. To do this, use the Plot Data function of LeafLab. This function will produce a scatter plot of your data.
- Interpreting Your Data Plots
Although basic trends in your data can sometimes be estimated by simply looking at the data points on your scatter plots, quantitative measures of the effects you are studying can only be determined by fitting a curve to your data. Curve fitting is an important part of studying correlations between data plotted on a scatter plot. Curve fitting involves producing a statistically derived best fit of data points and not a hand-drawn or estimated line connecting data points. LeafLab uses a statistical technique called least squares for estimating a best-fit line. See your instructor for additional help if you are uncomfortable or unfamiliar with curve fitting.
Once you have plotted your data, a "Plot #" tab will appear at the top of the Plot Data screen. Clicking on this tab will take you to the curve-fitting functions of LeafLab and allow you to switch between plots that you generate.
- Summary: What Did This Experiment Tell Us?
The experiment you just performed is representative of other experiments that you will conduct. A lot of information can be learned from studying the curves that you generate. Seek help from your instructor if you are unfamiliar with how to study a curve to interpret values on the x- and y-axis. Study the curve of PS Rates vs. Light in Tomato to answer the following questions (Hint: You may need to refer back to section e for definitions of each parameter of the plot):
Click on the Choose Leaf button on the left side of the screen. For this experiment we will use a leaf from a tomato plant. A dark box should appear around the tomato leaf in the left corner of this screen indicating that the tomato plant has already been selected (tomato is the default plant). Read the legend about tomato plants that appears on the right side of the screen. Similar legends will appear for each plant that you select. Also notice that the bottom three panels of this screen show graphics of the whole plant, leaf, and fruit structures for the tomato plant. Similar legends and graphics will appear for each plant you select. Review some of the other plants available in LeafLab by clicking on each plant button and reading the legends that accompany each plant.
Because the calculations of photosynthetic rate that this simulation will generate as data are expressed as a value per unit of leaf surface area, you must begin each experiment by determining the total surface area of the leaf you have chosen for your experiment.
Click on the Measure Area button on the left side of the screen. A view of a tomato leaf will appear, overlaid by a series of grid squares. Click on one of the squares. It will now be shaded green, indicating that you have measured this area of the leaf.
A tally of the number of squares selected and the total surface area (in cm2) for all squares selected is provided on the right side of the screen. The scale for measuring area is different for certain plants.
Notice that the scale for tomato plants is set up so that each square is 0.1 cm2 in area. Continue to select squares until you have measured the entire surface area of this leaf; however, when you get to the edges of the leaf, notice that some of the grid boxes are not completely filled by the leaf. For these boxes, double-click on each box. The box will now be shaded a lighter green than the other boxes. Notice that these boxes are scored as one-half of the area of the other boxes to adjust your area measurement for incompletely filled boxes. Hint: You can measure surface area quickly by using your mouse to click and drag across several boxes.
The surface area value that you just measured should be recorded in your lab notebook for future reference as follows:
Click on the Add to Notes button at the lower left of the screen to record this value in your notebook.
For each plant in LeafLab, only one size leaf will appear; therefore, once you have measured leaf area for a particular plant you will not have to measure the area of this leaf again unless you exit LeafLab and then return again. The area measurement will be available to you even if you switch plants to perform another experiment. If you do not measure leaf surface area at the beginning of an experiment, LeafLab will not let you run the experiment. Once you have defined the total surface area of the leaf, you are almost ready to run an experiment; however, before you can begin collecting data you must know what environmental parameters in the leaf chamber you can manipulate and the types of data you will be collecting.
Click on the Collect Data button on the left side of the screen. The screen that appears is labeled Input Controls. For this first experiment, we will leave many of the Input Controls at their default value. At the bottom of the screen, locate the box labeled "Expt #." This box will identify each experiment that you perform so you can return to this experiment if you need to. This first experiment should be indicated by a "1" in this box.
Find the input controls for each of the parameters mentioned in the preceding paragraph, then set up the conditions for this experiment as follows:
Set gas flow to a medium value by clicking on the "medium" button. Notice that gas flow has now changed from 0 ml/min to 500 ml/min.
Gas flow is a parameter that you will change depending on the size of the leaf you are working with. For tomato and goldenrod leaves, medium gas flow is appropriate. For corn, a high gas flow is necessary. For fescue, the small blades of grass require a low gas flow. If you try to run an experiment without gas flowing into the leaf chamber, no data will be generated when the lamp is turned on.
Leave temperature, carbon dioxide concentration, and the light filter at their default values. Unfiltered white light will be shining on the tomato leaf when we turn on the lamp intensity. It is best to turn on either the lamp or the gas flow as the last step in the experiment. Begin this experiment by leaving lamp intensity at 0mol/m2/s. Note: An intermediate value of approximately 1000
mol/m2/s is close to representing a typical value of sunlight on a sunny day. Temperature, CO2 concentration, and light intensity can be changed either by using the slider for each parameter or by entering a value into the text box that appears to the right of each slider.
Notice that once you turn on the lamp, a chart recording of CO2 output, as determined by the IRGA, will appear in a box just above the chart recording.
Locate the numerical value for CO2 output in the leaf chamber.
The IRGA is determining CO2 output by comparing the amount of CO2 entering the chamber with the amount of CO2 in the air leaving the chamber. Remember that the leaf is consuming CO2 as its cells perform photosynthesis but these cells are also undergoing cell respiration to produce ATP--a process that produces CO2 as a waste product. Therefore, the IRGA is recording CO2 output as a measure of net photosynthetic rate--the difference between CO2 consumption during photosynthesis and CO2 production during cell respiration. Note: It is important to realize that you are not measuring O2 production in these experiments.
To take measurements of CO2 output, click the Record button in the bottom right corner of the screen. When you do this, data on CO2 output will be recorded in tabular form in the Prepare Data view of LeafLab. Before recording any measurements, always make sure that the line on the chart recording is horizontal and not wavy before taking a measurement. This is especially important after you have changed an input parameter (because you must wait for the experimental conditions in the leaf chamber to "settle down" to your desired input setting(s)).
Click on the Record button to take a recording of CO2 output. Notice that when you click the Record button, a solid black line appears on the chart recording, indicating that a measurement was taken.
Continue this experiment by increasing light intensity by increments of approximately 200mol/m2/s to 200, 400, 600, 800, 1000, 1200, 1400, 1600, 1800, and 2000
mol/m2/s. It is easy to make these changes by typing these values into the text box to the right of the light intensity slider. After each change in light intensity, wait for the chart recording to flatten out to a horizontal line and then record your data by clicking the Record button.
Switch to the Prepare Data view by clicking on the Prepare Data button at the left of the screen.
The purpose of the Prepare Data function is to use the data that you recorded to calculate photosynthetic rate. In this view, the table at the bottom of the screen contains values for all of the input parameters of each experiment that you conducted. This table also indicates the concentration of CO2 entering the leaf chamber (C-in) and the concentration of CO2 leaving the leaf chamber (C-out). The calculated value for photosynthetic rate (P) will appear in the far right column of the table. LeafLab will calculate P for us in a moment.
The four equations at the top of this screen will be used to calculate photosynthetic rate. Each equation is described below.
Equation 1: Calculates CO2 consumption by subtracting C-out from
C-in and reports this value as the change in CO2 concentration
(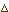 CO2).
CO2).
Equation 2: Converts 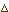 CO2 concentration from parts per million to
CO2 concentration from parts per million to
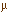 mol/liter, based on the temperature of the leaf flask.
mol/liter, based on the temperature of the leaf flask.
Equation 3: Calculates the rate of CO2 that is available for
exchange between the leaf and the flask by multiplying 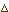 CO2
concentration against gas flow.
CO2
concentration against gas flow.
Equation 4: Net photosynthetic rate (P) is calculated by
dividing the CO2 available for exchange by the total surface area
of the leaf being studied. This value is reported as the number of
micromoles of CO2 released per square meter of leaf surface area
per second (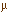 mol/m2/s).
mol/m2/s).
Look at the table and review the information contained in each column before calculating photosynthetic rate.
To enter data into these equations, click on the row for the experiment that you want to analyze-in this case, experiment 1. The selected row will now be highlighted in green. Select the whole table by clicking on the top row (0 light data) and dragging the mouse to the bottom of the table. To perform the calculations, click on the Compute button, located on the right side of the screen just above the P column in the table. Net photosynthetic rate will now be calculated and added to the table.
Click on the Plot Data button on the left side of the screen. Click in the title box at the top of this screen and title this first plot "PS Rates vs. Light in Tomato" or another appropriate name of your choice.
You can express your data on these plots in two different ways. On the x-axis you can choose to plot either "light intensity" (this is the default) or CO2 input (C-in). On the y-axis you will plot photosynthetic rate (P) values. You can also change the symbol and symbol color that will appear on the plot. Prepare a plot of light intensity versus P values as follows:
Start at the top row of data in the table, and plot the entire table of P values by holding down the Shift key and clicking on each row of data until you get to the last row of the table. The entire table should now be colored green, indicating that you selected all of the data in the table. Leave the x-axis at its default value of light intensity. Click the Plot Selected Data button to plot the data. A plot of your data should appear. Notice that the horizontal line at the center of the plot indicates a "0" value of light intensity.
Add the data from this plot to your notebook by clicking on the Add to Notes button at the lower left of the screen.
Click on the Plot 1 tab to enter the curve-fitting view.
An enlarged view of the plot should now appear with a series of curve-fitting controls to the left of the plot. The purpose of each control is described below.
Curve - generates a best-fit curve based on the data points selected.
y -Intercept - indicates the rate of dark respiration (light compensation point)
Slope (of the line) - photochemical efficiency; the rate at which photosynthesis increases as light intensity increases.
Asymptote (where the curve forms a straight line indicating that the data has leveled off) - indicates photosynthetic saturation (maximum rate of photosynthesis).
Error SS - error sum of squares; based on the calculations of the least squares parameter estimation of a best-fit line. Error SS is an expression of the least squares calculation of the (sum and squared) distances of each data point from the fit line.
When fitting a curve it is helpful to proceed as follows:
(1) Change the intercept first. To do this, return to the data table by clicking on the Data tab. Look at the zero light measurement in the table and use the P value for this measurement as the initial measurement of the intercept. Return to the curve-fitting view and enter this P value directly into the intercept box.
(2) Look at the plot and form a rough estimate of where the data levels off. This is the asymptote. To determine this value, click on the plot next to the data point that you think represents the asymptote. Two sets of numbers in parentheses will appear. The first number is light intensity (in nanometers) and the second number is photosynthetic rate (P). Enter this P value into the asymptote box.
(3) Next, increase the slope of the line until the curve looks like it is matching (fitting) the data points. Do this by clicking the up arrow next to the slope function (you will see the line rise up and begin to form a curve). Make adjustments to the slope of the curve and asymptote as necessary to achieve the best-fit curve for the data points.
(4) Look at the value for the Error SS. Adjust the slope, asymptote, and intercept to minimize this number. Use the up or down arrows next to each parameter to adjust these values. If you make a change to one parameter, you will need to check the other two parameters to see if further changes are necessary. Stop only when any further changes to all three parameters increase the Error SS value. The values that give the smallest Error SS (the least squares parameter estimates) produce the best-fit line for your data points.
(5) Save your plot by clicking on the Export Graph button at the left of the screen. A separate window will now open showing your plot and a table with the intercept, slope, asymptote, and Error SS values. You can print this page by clicking the print button on your Web browser, or you can save this page to a disk by going to File and using the Save As feature of your browser.
What is the relationship between an increase in light intensity and photosynthetic rate in tomato leaves? Does this relationship support the hypothesis that you formulated?
Photosynthetic saturation is the maximum rate of photosynthesis. What was the value for photosynthetic saturation in tomato leaves? What value of light intensity produced photosynthetic saturation in tomato leaves? Based on what you know about photosynthesis, provide possible reasons for what causes photosynthetic saturation (these cannot be determined from the plot).
Follow the steps detailed in the first experiment to test the effects of an increase in light intensity on photosynthetic rates in corn (a C4 plant). The only modification to the experiment is that you will need to use a high rate of gas flow. Keep all other parameters the same as you did for tomato. (Note: When calculating P and plotting your data, make sure that you select only those values that you recorded for corn and not previously recorded values for tomato.) Plot photosynthetic rate versus light intensity and fit a curve to the data as you did for tomato, then answer the following questions:
What is the relationship between an increase in light intensity and photosynthetic rate in leaves from a corn plant? How does this relationship compare with what you observed for tomato plants?
Photosynthetic saturation is the maximum rate of photosynthesis. What value of light intensity produced photosynthetic saturation in corn leaves?